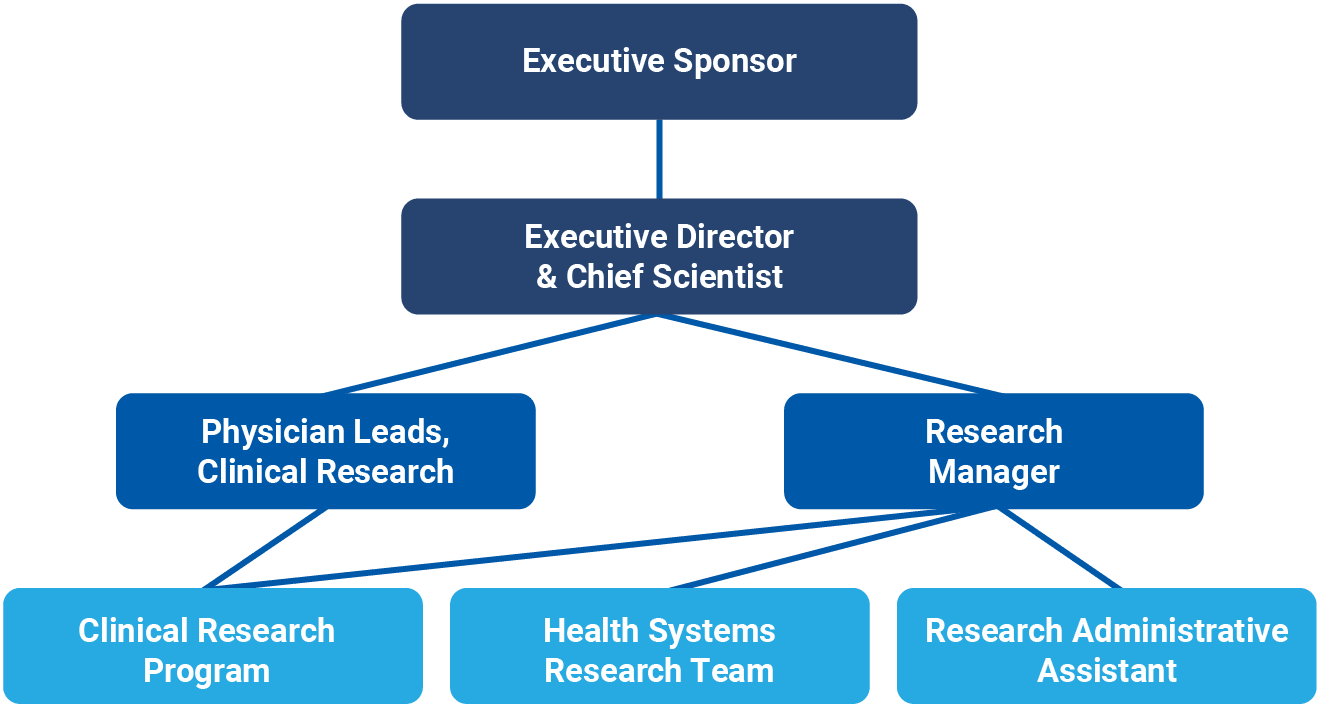
Dr. Jennifer Tsang (she/her), MD, PhD, FRCPC, ABOM, completed her Doctorate of Medicine Degree (Cum Laude) at the University of Ottawa in 2003. She returned to Toronto where she completed her residency training in Internal Medicine in 2006 followed by fellowship training in Adult Critical Care Medicine in 2008. While working as an adult critical care physician at Sunnybrook Health Sciences Centre in Toronto, she completed her PhD study at the University of Toronto in the field of molecular biology to understand the biology of sepsis. In 2013, she joined Niagara Health as an adult critical care physician and in 2015, she was appointed as the Physician Research Lead. In 2020, she received designation as an American Board of Obesity Medicine Diplomate for the provision of specialized care to patients with obesity in Southern Ontario. She is currently the Executive Director and Chief Scientist of the Niagara Health Knowledge Institute; an Associated Professor in the Department of Medicine, McMaster University; and the Regional Deputy Research Director, Internal Medicine Residency Program, McMaster University. She founded the Critical Care Research Program at Niagara Health, a program that was recognized by Health Standards Organization as a Leading Practice. She has over 95 peer-reviewed research publications and over $85 million in research grant funding as Principal Applicant and Co-Applicant. She received the McMaster University Department of Medicine Early Career Research Award in 2015, Canadian Critical Care Trials Group Early Career Research Award in 2018 and the McMaster University Department of Medicine Mid-Career Research Award in 2020.
Her academic work focuses on health research transformation in Canada by increasing research capacity in community hospitals across Canada. She is active in expanding clinical trials to Niagara Health and other community hospitals across Canada and is co-investigator of over 20 multicentre clinical trials. She is heavily involved at the provincial level and national level. At the provincial level, she sits on the planning committee of the Critical Care Services Ontario’s Critical Care Clinical Practice Rounds. She also sits on the Ontario Hospital Association’s Hospital-Based Coordination Working Group with a vision to develop a fully integrated health research and healthcare delivery system across Ontario. At the national level, she is the co-founder and co-chair of the Canadian Community ICU Research Network and the co-chair of the COVID-19 Network of Clinical Trials Networks Community Acute and Critical Care Working Group with the mandate to provide financial support and mentorship to community hospitals in developing research programs. She is a Council Member of the Royal College Physicians and Surgeons of Canada and a member of the Royal College Research and Evaluation Advisory Committee. She is a Board Member of the Canadian Critical Care Trials Group. She is also active in educating and equipping health and research professionals in a broad range of research methodologies through supervising graduate students and postdoctoral fellows, and through her involvement as a steering committee member and mentor of the innovative CIHR-funded Life-Threatening Illness National Group (LifTING) Research Training Platform with the mandate to address the lack of translation of new scientific knowledge into improved healthcare practices and the ongoing health inequities despite significant advances in health sciences. She is a co-investigator of the CIHR-Funded Health Systems Impact Research Training Program. Outside of her clinical and academic work, she served as a Board Member at Quest Community Health Centre in Niagara from 2019-2024 and she was the Co-Chair I from 2023-2024.
Internationally, she serves on the Platform for Developing Precision Approaches to Interpandemic and pandemic Trials, PRIME Ireland, the Patient Partner Involvement and Engagement Working Group for the Global Federation of Platform Trials, the InFACT/ISARIC Global Acute Care Research Collaboration, and the World Health Organization’s Global Optimization of Respiratory Support Collaboration Working Group.